How To Find Total Atoms
Solved:calculate the number of atoms, molecules, or formula units that Atoms number molecules calculate formula mass units large Atoms formula counting chemical count equations if me
Counting Atoms in a Chemical Equation - YouTube
Atoms and atomic structure Atom atoms molecule ion represents determining 1.6 periodic variations in element properties – inorganic chemistry for
Atoms nh3 approximately sarthaks
Atoms gramsCounting atoms in a formula – chemsimplified Atoms molecules calculate formulaRadius radii periodic electron properties electrons atomic shielding covalent atoms molecules valence halogen zeff configurations diatomic ions libretexts molecule pageindex.
Moles to atoms calculatorNumber atoms calculate present each Match each diagram to the atom or ion it representsCounting atoms in a chemical equation.

Atoms atom quizlet particles called indivisible radioactivity wpclipart islam previously
The number of atoms in 4.25 g of nh3 is approximatelyAtoms moles molecules avogadro calculating 3.1: types of chemical compounds and their formulasAtoms & molecules: e-chapter — the biology primer.
Atoms molesCounting the number of atoms in a molecule Compounds chemical types formulas their chemistry molecules elements covalent chem oxygen each molecule atomsSolved:calculate the number of atoms present in each of the following.

Neutrons electrons atoms protons molecules atom proton nucleus subatomic particle biology nuclei fundamental composed hydrogen charged neutrally chemistry heavier
Solved:calculate the number of atoms, molecules, or formula units thatAtoms number molecule counting Atoms number sample calculating chemistryHow many atoms make up an element.
Atoms chemical equation counting .

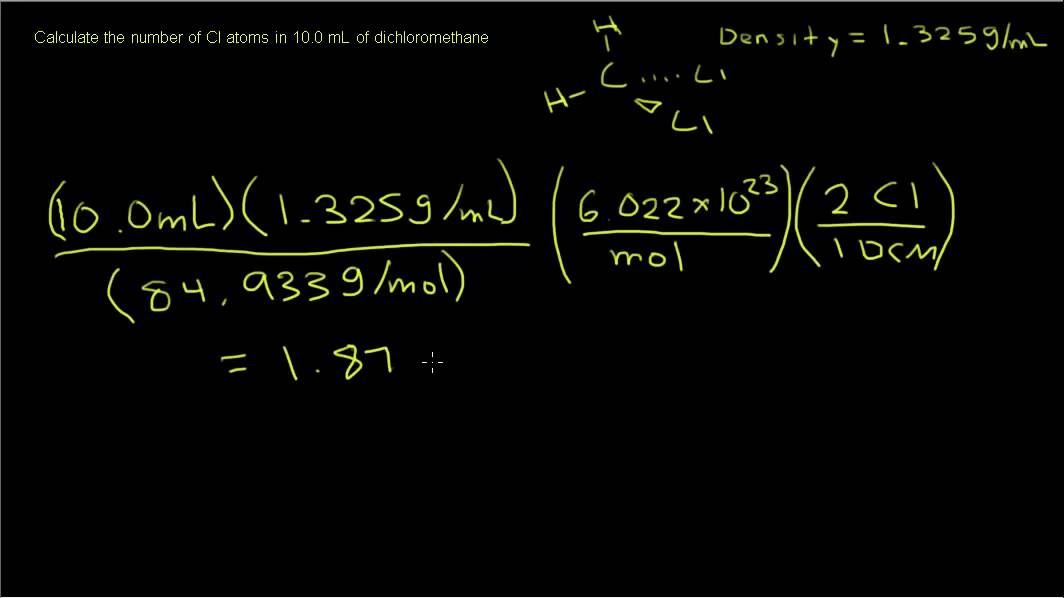
Chemistry - Calculating Number of Atoms in a Sample - YouTube

Atoms & Molecules: e-chapter — The Biology Primer

Moles to Atoms Calculator - Calculator Academy
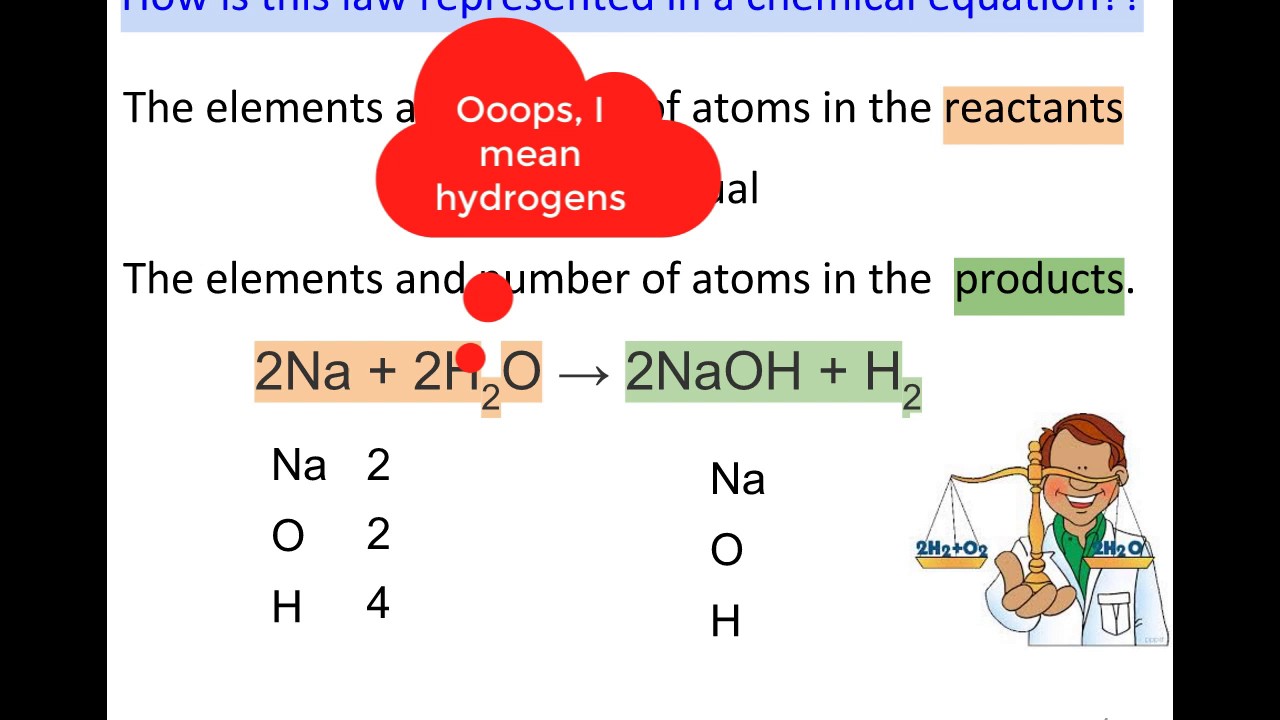
Counting Atoms in a Chemical Equation - YouTube

SOLVED:Calculate the number of atoms, molecules, or formula units that

Counting Atoms in a Formula – ChemSimplified
The number of atoms in 4.25 g of NH3 is approximately - Sarthaks
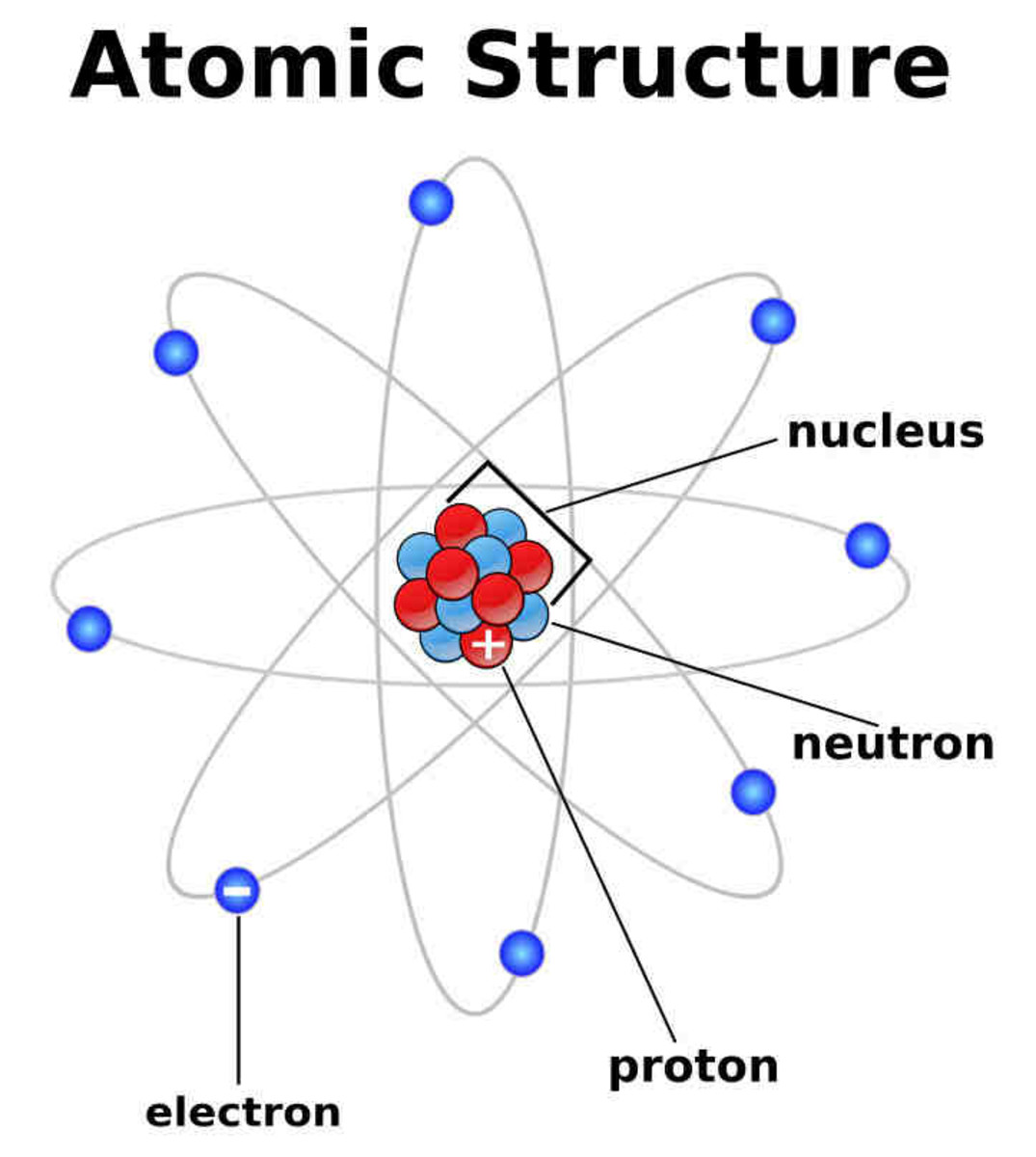
Atoms and Atomic Structure - HubPages

SOLVED:Calculate the number of atoms present in each of the following
