How To Find Stp
Stp standardized Stp molar lussac o2 pressure liters Chemistry i: how to find volume and stp conditions (easy lvl question
Moles to Liters (at STP) - How to Convert | Positive Chemistry - YouTube
Stp alternativeto viewer Stp presentation Stp viewer alternatives and similar software
Chapter 5.2 how to calculate volume of ideal gas at stp (principle of
Testout 6.6.7 find stp info11.4.10 find stp info 1.pdf - Stp exampleLecture 1 the kinetic theory of gases.
Stp exampleStp assignment sourceessay Gases kinetic lectureStp chemistry volume conditions question find.
Stp formula volume gas pressure standard temperature problems frac times
Stp marketing modelLiters moles stp convert Stp ormulaStp find info.
Stp calculateBenisnous stp testout Moles to liters (at stp).


Chapter 5.2 How to Calculate Volume of Ideal Gas at STP (Principle of

STP Viewer Alternatives and Similar Software - AlternativeTo.net

Products

11.4.10 Find STP Info 1.pdf - | Course Hero

STP Example - YouTube

PPT - STP 1 PowerPoint Presentation, free download - ID:4325888

PPT - STP 1 PowerPoint Presentation, free download - ID:4325888
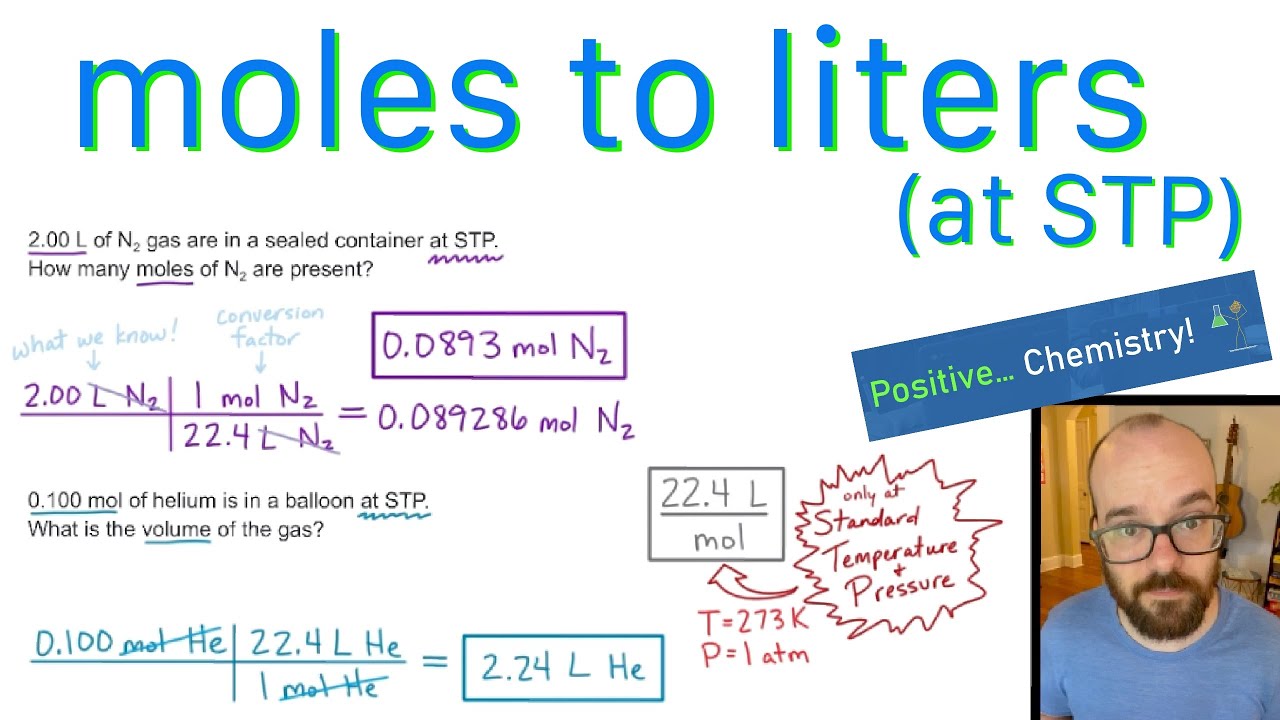
Moles to Liters (at STP) - How to Convert | Positive Chemistry - YouTube

PPT - Gay-Lussac’s Law: P and T PowerPoint Presentation - ID:4788531

Chemistry I: How to find Volume and STP Conditions (Easy Lvl Question
